Abstract
Context and objective
Intensive chemotherapy (IC) remains an important therapeutic option in HR-MDS, but in-depth evaluation of response to IC remains ill studied. We previously reported results of CPX-351 in 31 untreated higher risk MDS patients (pts) (GFM-CPX-MDS study, NCT04273802, ASH 2021 Abst #243). Responses were associated with a significant decrease of CD34/CD117 progenitors and restoration of normal hematopoiesis patterns in bone marrow (BM) samples in multiparameter flow cytometry.
In this ancillary study, the aim was to evaluate molecular responses by high-throughput sequencing (HTS) centrally. This involved description of the pts molecular landscape and an assessment of variant allele frequency (VAF) variations after induction treatment with CPX-351.
Methods The mutational landscape was explored in BM nuclear cells by capture HTS of 42 genes (SureSelect XTHS2 Agilent) with unique molecular identifiers (UMI), unique dual index (UDI) and Illumina paired-end sequencing (Miseq and Nextseq). Somatic mutations (SM) were considered significant for VAFs >2%. Molecular response was evaluated by comparing baseline and post-induction. Molecular clearance was defined according to (i) clearance (<2%) of all SM excluding the "pre-leukemic” SM of DNMTA, TET2, ASXL1 (DTA) (ii) clearance of all non-DTA SM < HTS detection threshold (iii) normalized VAF decrease of the major non-DTA baseline SM (nmVAF).
Results Median age of the 31 pts included was 62 years (range 31-69). There were 26 MDS-EB2 and 5 CMML-2. IPSS was int-2 in 26 pts and high in 5. Cytogenetic IPSS-R was very good (6.5%), good (71%, including normal in 22 pts), int (9.5%), poor (6.5%) or very poor (6.5%). After induction with CPX-351 (3 days cycle and a possible 2-day second induction cycle for non-responders), 16 pts (52%) reached CR, 4 (13%) CRi, 7 (22%) MLFS while 4 (13%) were in stable disease according to ELN 2017 criteria (which appeared more adequate than MDS IWG 2006, as reported at ASH 2021, Abst #243). Median follow-up was 511 days (179-710), 29/31 patients (94%) received allogeneic hematopoietic stem cell transplantation (Allo-HSCT) after, no, 1, 2, 3 or 4 CPX-351 consolidation cycles (that included only 1 day of CPX-351) in 10, 16, 7, 3 and 1 patients respectively. So far, 4 relapses have occurred, including 3 in transplanted pts.
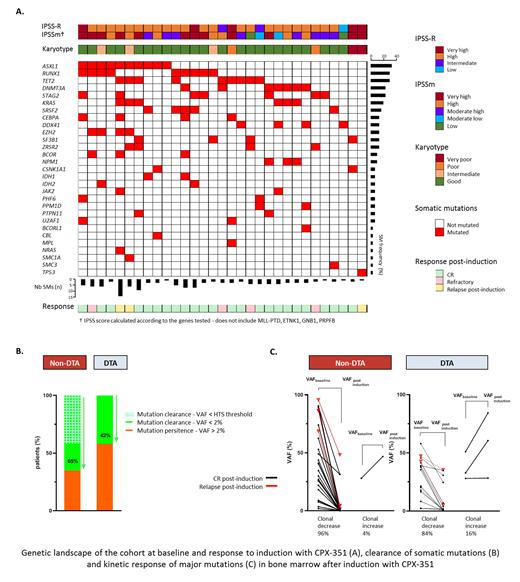
At inclusion, all pts had at least one mutation (median 3, [1-14]) with a mean VAF of 30% (2-89), implicating at least one DTA (71%; 22/31) or one non-DTA genes (93%; 29/31) (fig). Very high or high risk was seen in 19/31 (61%) pts according to the new IPSSm classification.
Post induction molecular response was evaluated at a median of 53 days from CPX-351 onset (28-112) in 26 of the 27 who reached at least MLFS, and had a post-induction sample. Molecular clearance was observed (i) at the 2% threshold of non-DTA genes in 17/26 (65%) pts (ii) at the HTS detection threshold (0.1% i.e. minimum considered 3X with median coverage > 3000X) in 11/26 pts (42%) (iii) by nmVAF decrease in 25/26 pts (96%) with a median of -62% (-2 to -100%) (fig) while the remaining pt had an increase of SRSF2 (+67%) and RUNX1 (+46%) SMs but disappearance of three STAG2 SMs and was still in CR after allogeneic SCT. Of note, DTA SMs remained detectable in all pts with a median nmVAF modulation of -86% (+83% to -99.6%, fig) from baseline to post-induction.
Of the 4 non-responders to CPX-351, 2 had high risk anomalies (inv(3) and EZH2 / RUNX1 SMs). The 3 relapses observed after alloHSCT, at 7, 8 and 15 months had nmVAFs decrease from baseline to post-induction of -95%, -98% and -50% respectively. Among them, one had a very complex karyotype and a homozygous TP53 mutation, the two others had a high mutational load and EZH2 mutations and one of the latest was transplanted in refractory state.
Clonal evolution was observed in 5/26 pts with significant emergence of new mutations involving PPM1D, ASXL1 or JAK2 and in one pt the emergence of EZH2, RUNX1 and TET2 SMs. Four of these pts remained in morphological CR while one progressed to AML.
Conclusion In 25/26 CPX-351 responders, a significant decrease of nmVAF was observed, confirming the interest of CPX-351 treatment in higher risk MDS, especially as a bridge to transplant. Follow up is ongoing and few relapses were seen to evaluate the predictive role of molecular analysis. An update will be presented at the meeting.
Disclosures
Chevallier:Takeda: Honoraria; Jazz Pharmaceuticals: Honoraria; Incyte: Research Funding; Pfizer: Research Funding; Abbvie: Honoraria. Gourin:AbbVie: Other: Fees for advisory boards; Blueprint Medicines Corporation: Other: Fees for advisory boards. Dumas:Astellas: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; JAZZ: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees. Park:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sandoz: Research Funding. Cluzeau:Syros: Speakers Bureau; Pfizer: Other: international congress; Abbvie: Consultancy, Speakers Bureau; Jazz Pharma: Speakers Bureau; Celgen/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Agios: Speakers Bureau; Incyte: Speakers Bureau; Astellas: Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: international congress, Speakers Bureau. Fenaux:Novartis: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Celgene/BMS: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Syros Pharmaceuticals: Honoraria.
OffLabel Disclosure:
CPX-351 in Higher Risk Myelodysplastic Syndromes
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal